Refer a patient | Questions? (916) 487-8230
Sponsors / CROs
- The CTR Team
- Study Background (260+)
- Therapeutic Areas
- Population & Reach
- Facilities
- Key reasons for selecting CTR
Contact Us
We will get back to you as soon as possible
Please try again later
-
Jeffrey D. Wayne, MD
Curriculum VitaePrincipal Investigator
-
Sharmila Chattergee, MD, MPH
Curriculum VitaeSub-Investigator
-
Joseph Hobbs, PA-C
Curriculum VitaeSub-Investigator
-
Leesa Koskela, CCRC
Curriculum VitaeAssociate Clinical Director
-
Esther Gayoba, PA
Curriculum VitaeSub-Investigator
Why Select CTR?
- 300+ completed Phase 2, 3 and 4 trials since 1992;
- 100% research focused Principal Investigator since 2010;
- Large team including 3 Sub-Investigators, 4 Study Coordinators, 2-member\dedicated recruitment department and support staff;
- Adjoining medical practice for which Dr. Wayne serves as supervising Physician;
- Comprehensive Physician Principal Investigator oversight including creation of source documents for all studies, frequent daily meetings with Study Coordinators and Recruitment team as well as new study startup (feasibility questionnaire and assessment, contract/budget, regulatory);
- 19,000 patient database (CTR + Lincoln Family Practice);
- Highly refined recruitment processes and dedicated resources;
- Broad therapeutic area coverage;
- Able to accommodate studies requiring blind and unblinded staff;
- Ability to perform medication infusion and injection studies, extended site visits such as serial lab draw and serial spirometry;
- CLIA certified labs (both sites);
- Labs (local and central);
- Secure and temperature controlled drug rooms;
- Imaging (comprehensive);
- IRB approved Pre-Screening ICFs (Spirometry, HbA1c, BP, Lipid, ALT, PSA, eGFR and UACR);
- Extensive hands-on experience with major IRT/EDC systems (ClinTrak, Fusion Axiom, IBM, Inform, Medidata Rave, Oracle);
- Specialty Services (Sub-Investigators and Consultants);
- Established and active SOPs (regularly updated);
- Current GCP and IATA certifications.;
- In good standing with ACRP;
- FDA Regulated Business.
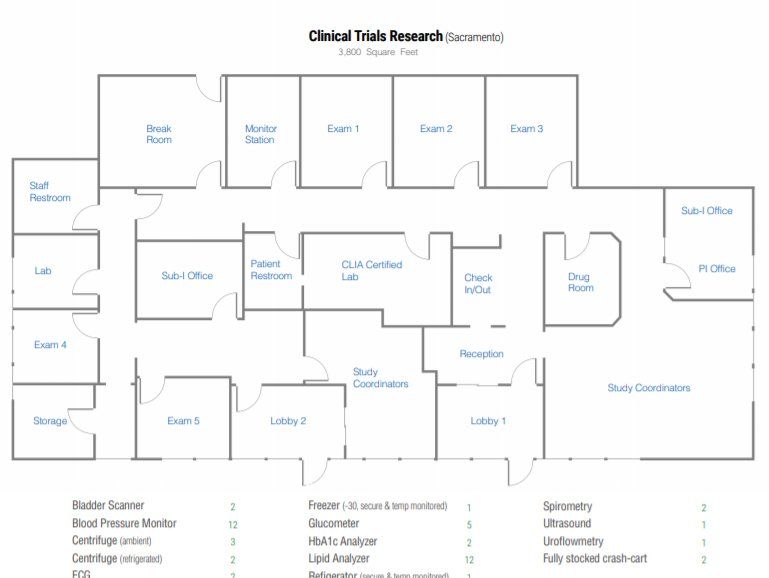
Our Medical Facilities: Lincoln & Sacramento
About Clinical Trials Research
Clinical Trials Research ® (CTR) has two research sites providing coverage to the greater Sacramento area in northern California. Our medical director, Jeffrey D. Wayne MD has served as a Principal Investigator since 1992 overseeing more than 300 phase 2, 3 and 4 studies. Since 2010, Dr. Wayne has been focused 100% on pursuing excellence in clinical research.
CTR brings the experience, the team, the facilities, the dedication and the reaction time to positively impact your study enrollment objectives.
If you are tasked with identifying high performing sites for your current and upcoming studies, give Clinical Trials Research the opportunity to demonstrate how our team can be a solid partner in study trial research.
Where to Find Us
160 Gateway Dr. Suite 100
Lincoln, CA 95648
Monday - Friday 8:00 AM - 4:30 PM
(916) 487-8230
Ⓒ Copyright 2020 Clinical Trials Research. All Rights Reserved.
Need immediate assistance? Call (916) 487-8230 from 8:00 am to 4:30 pm PST Monday through Friday.